lateral flow assay covid
To address this need serologic lateral flow assays LFAs which can be easily. Using a lateral flow assay LFA testing platform the seropositivity in 63 New York Blood Center NYBC Convelescent Plasma CP donor samples were evaluated for the presence of COVID19.
Biosensors Free Full Text Recent Advances In Novel Lateral Flow Technologies For Detection Of Covid 19 Html
A lateral flow assay is a rapid diagnostic capable of providing quantitative semi-quantitative or qualitative results in minutes.

. Buy online and delivered to your door. To combat the enduring and dangerous spread of COVID-19 many innovations to rapid diagnostics have been developed based on proteinprotein interactions of the SARS-CoV-2 spike and nucleocapsid proteins to increase testing accessibility. Lateral flow tests for COVID-19 are more commonly referred to as rapid tests kits and have been placed in huge demand as they have been made available to the general public in most parts of the world.
The test usually involves taking a sample from your throat and nose or from your nose only using a swab. Designed for complete in-the-field use. From 1st April free NHS lateral flow tests are no longer available - but dont worry well continue to offer lateral flow test kits in various pack sizes including single tests for just 189 or up to a pack of ten for 17 170 per test.
COVID-19 lateral flow tests rapid diagnostic tests help to test the virus that causes the infection quickly. Every time you use a. Lateral flow test for COVID-19.
A lateral flow assay LFA platform is a powerful tool for point-of-care testing POCT especially for self-testing. Sensitivity is a key parameter for reliable and accurate detection. It shows you the result on a handheld device that comes with the test.
Although the LFA platform provides a simple and disposable tool for Coronavirus disease of 2019 COVID-19 antigen Ag and antibody Ab screening tests the lower sensitivity for low virus titers has been a bottleneck for practical applications. Der Lateral-Flow-Test auch Lateral-Flow-Assay englisch für seitlicher Flusstest ist eine biochemische Methode zum qualitativen Nachweis von Stoffen z. You get a result in 10 to 30 minutes depending on the type of rapid lateral flow test you.
When the researchers used a new formula for calculating the rapid tests accuracy they found LFTs were more than 80 effective at. A rapid lateral flow test is a coronavirus test you do yourself. You reach for one of those lateral flow tests LFT youve got stashed.
Developing lateral flow COVID-19 assays isnt without a few pressing technical challenges that the diagnostics team is currently addressing. These antigen tests have most prominently been developed using the lateral flow assay LFA test platform. A lateral flow assay LFA platform is a powerful tool for point-of-care testing POCT especially for self-testing.
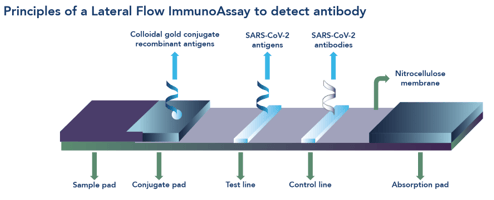
LFAs are amenable for home testing and community seroprevalence monitoring efforts. Ad RD through final product. The murine S96 monoclonal antibodies are prefixed onto the test line T of the lateral flow strip and the control line C is.
Results within 15 minutes. 23 hours agoAmid the COVID-19 crisis the global market for Lateral Flow Assays estimated at US78 Billion in the year 2020 is projected to reach a revised size of US112 Billion by 2026 growing at a CAGR. A swab is taken from the back of the nose or throat mixed with an extraction fluid and a drop of this mixture is placed.
Design and operation of the HC-FIA assay. LFTs are a rapid way of testing for Covid-19. So were developing our antigen detection test using europium particles that.
The need for population seroconversion data is apparent to monitor and respond to the pandemic. 23 hours agoAmid the COVID-19 crisis the global market for Lateral Flow Assays estimated at US78 Billion in the year 2020 is projected to reach a revised size of US112 Billion by 2026 growing at a CAGR. You wake up with a pounding head sore throat and runny nose.
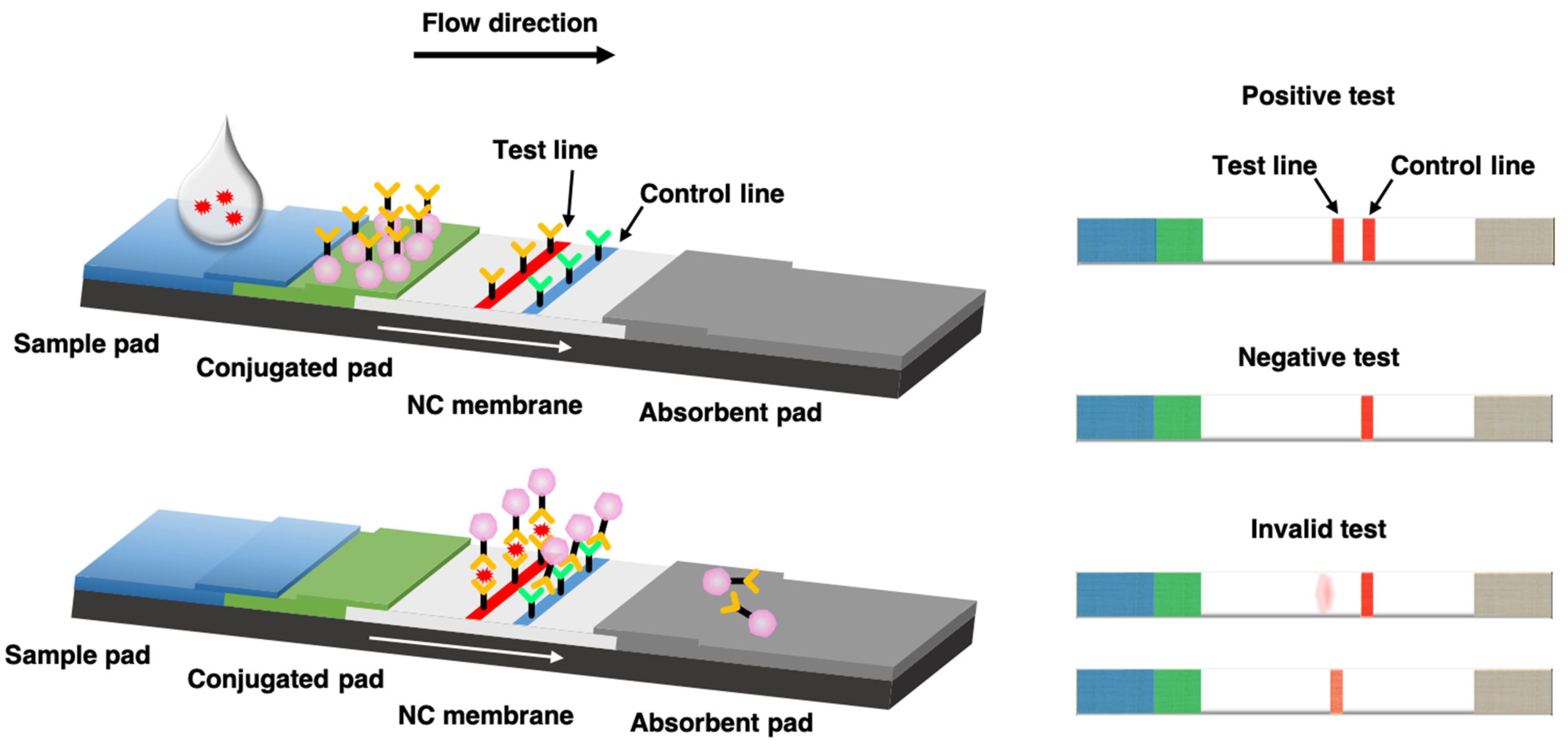
If the viral proteins are present they will show up in a colored line similar to a positive. Test auf Antikörper IgG und IgM links negativ rechts positiv. To combat the enduring and dangerous spread of COVID-19 many innovations to rapid diagnostics have been developed based on proteinprotein interactions of the SARS-CoV-2 spike and nucleocapsid proteins to increase testing accessibility.
Although the LFA platform provides a simple and disposable tool for Coronavirus disease of 2019 COVID-19 antigen Ag and antibody Ab screening tests the lower sensitivity for low virus titers has been a bottleneck for. Rapid diagnostic tests retrieve samples from the nose throat to detect the viral proteins antigenrelated to the COVID-19 infection. The coronavirus disease of 2019 COVID-19 pandemic caused by infection with the severe acute respiratory syndrome coronavirus-2 SARS-CoV-2 has undoubtedly resulted in significant morbidities mortalities and economic disruptions across the globe.
These antigen tests have most prominently. What is a lateral flow test. Development of your entire lateral flow assay from concept to commercialization.
As mentioned the popularity of lateral flow tests have been renewed with COVID-19. These antigen tests have most prominently been developed using the lateral flow assay LFA test platform. Evaluation of LFAs includes both laboratory assessment of performance characteristics and fitness for implementation.
We obviously want the tests to be highly sensitive. Abstract To combat the enduring and dangerous spread of COVID-19 many innovations to rapid diagnostics have been developed based on proteinprotein interactions of the SARS-CoV-2 spike and nucleocapsid proteins to increase testing accessibility. 1 2 3 Der lateral flow test ist eine.
Amid the COVID-19 crisis the global market for Lateral Flow Assays estimated at US78 Billion in the year 2020 is projected to reach a revised size of US112 Billion by 2026 growing at a CAGR. Protein-based lateral flow assays for COVID-19 detection. Objective COVID19 has caused a global and ongoing pandemic.
Lateral flow assays LFAs are affordable and easy-to-use serologic assays for SARS-CoV-2.

A Point Of Care Lateral Flow Assay For Neutralising Antibodies Against Sars Cov 2 Ebiomedicine

A Point Of Care Lateral Flow Assay For Neutralising Antibodies Against Sars Cov 2 Ebiomedicine
The Rise Of The Lateral Flow Test Everything You Need To Know About Lateral Flow Tests In Five Steps Una Health
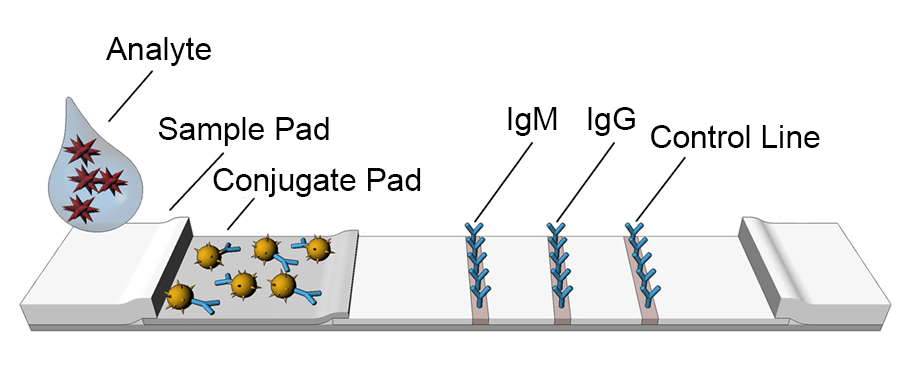
What Is A Lateral Flow Assay And How Does It Work Joysbio Biotech
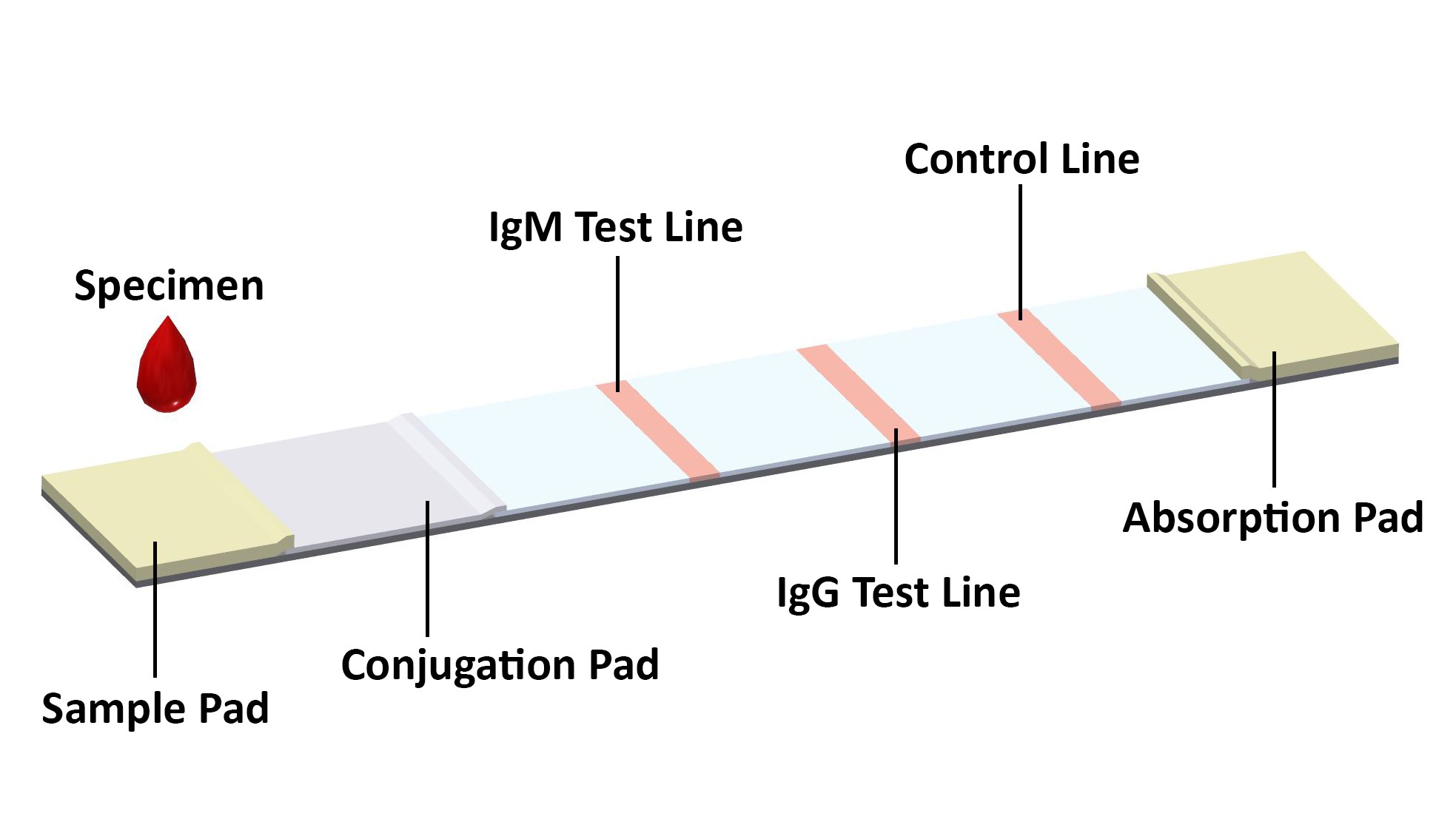
Covid 19 Antibody Rapid Test Kit Coronavirus Igg Igm Rapid Test
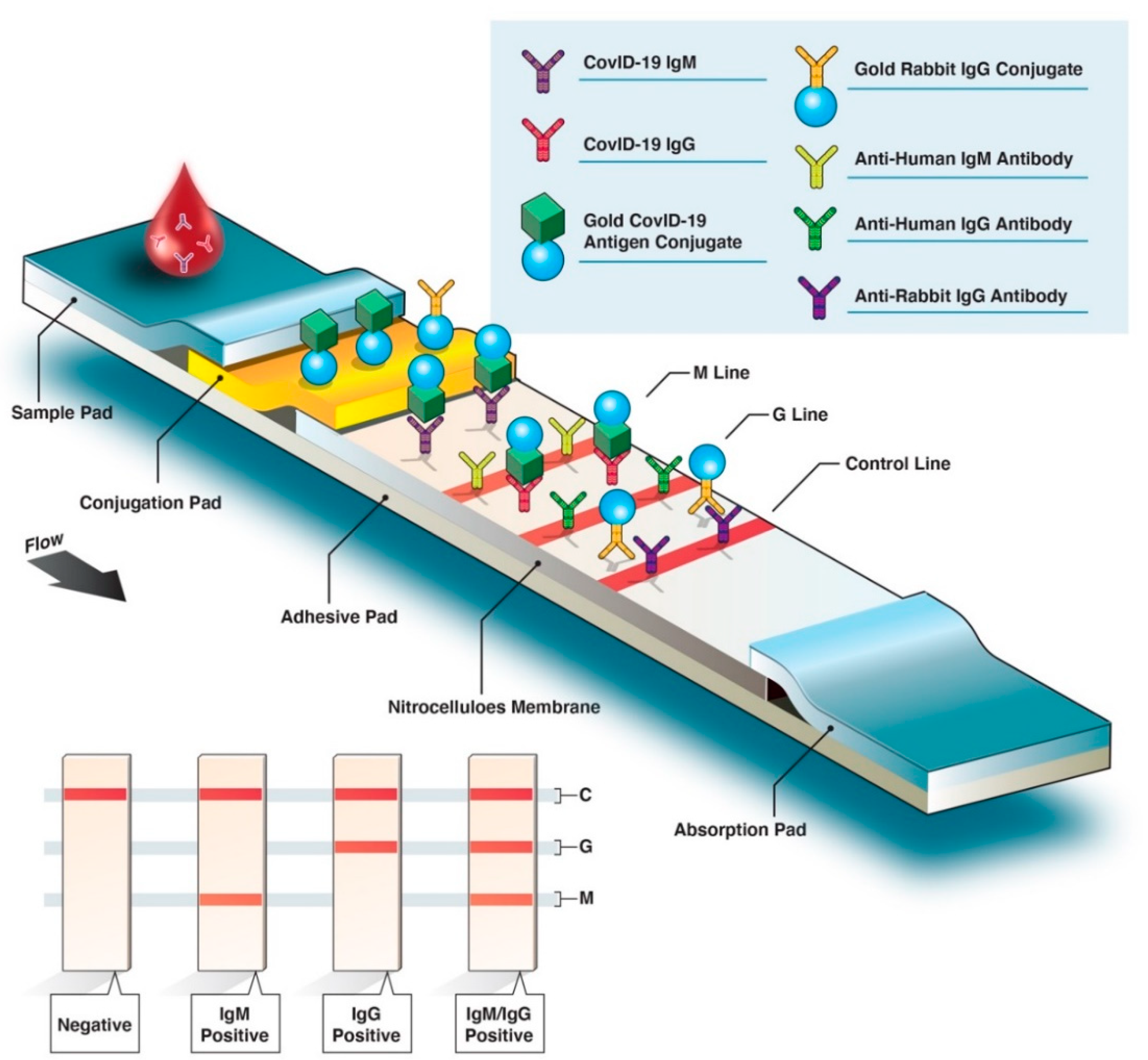
Diagnostics Free Full Text Covid 19 Serological Tests How Well Do They Actually Perform Html

Lateral Flow Assay Labels And Conjugation Technologies

Coronavirus Covid 19 Sars Cov 2 2019 Ncov Assay Kits Lateral Flow Immunochromatography Products Mybiosource
![]()
Using A Lateral Flow Device Lfd To Test For Covid 19 Key Points To The Correct Technique On Vimeo
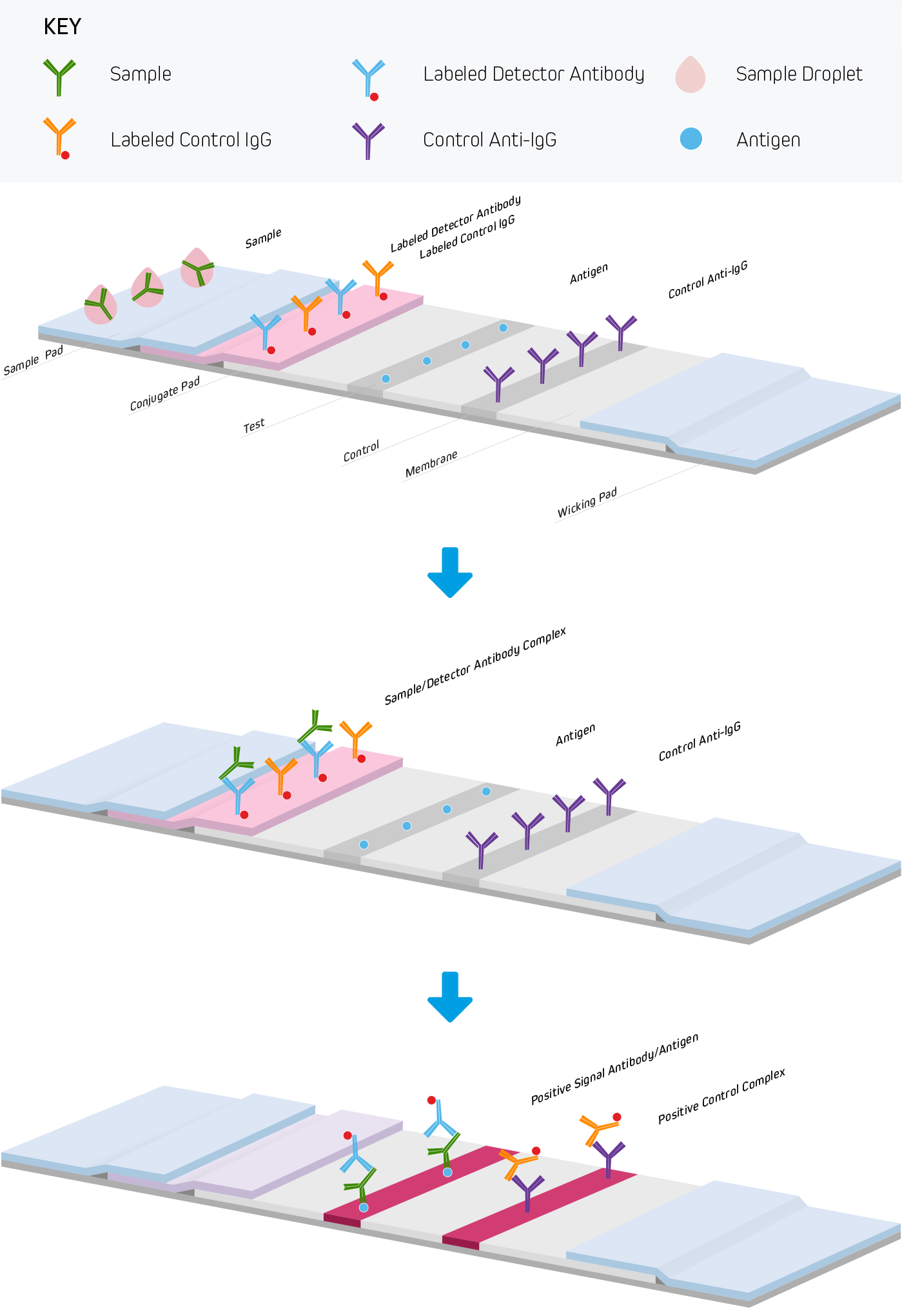
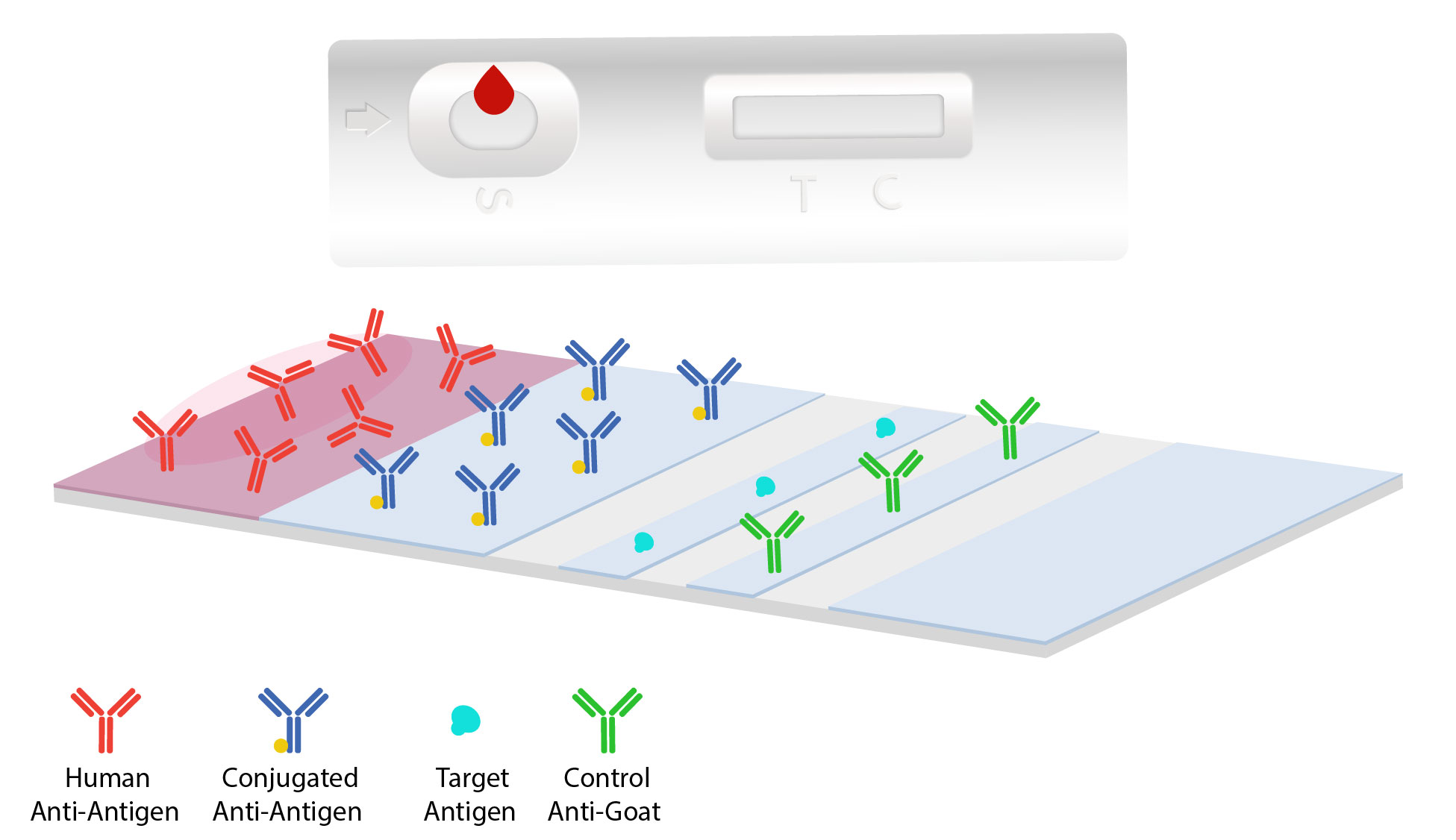
Lateral Flow Immunoassays Jackson Immunoresearch

Covid 19 Lateral Flow Self Test 1 92 Per Test Stock Available
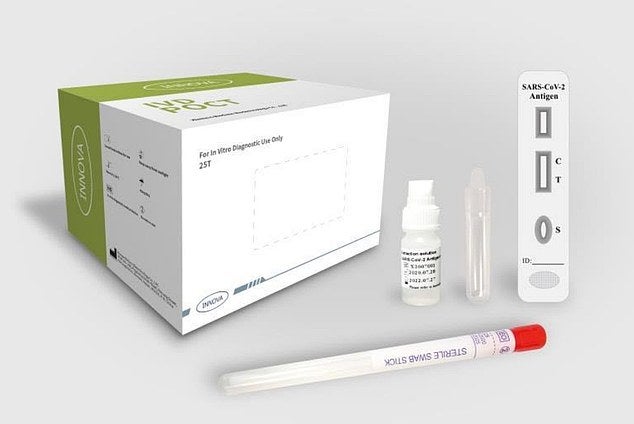
The Uk Is Trialling Lateral Flow Testing For Covid 19 How Does It Work
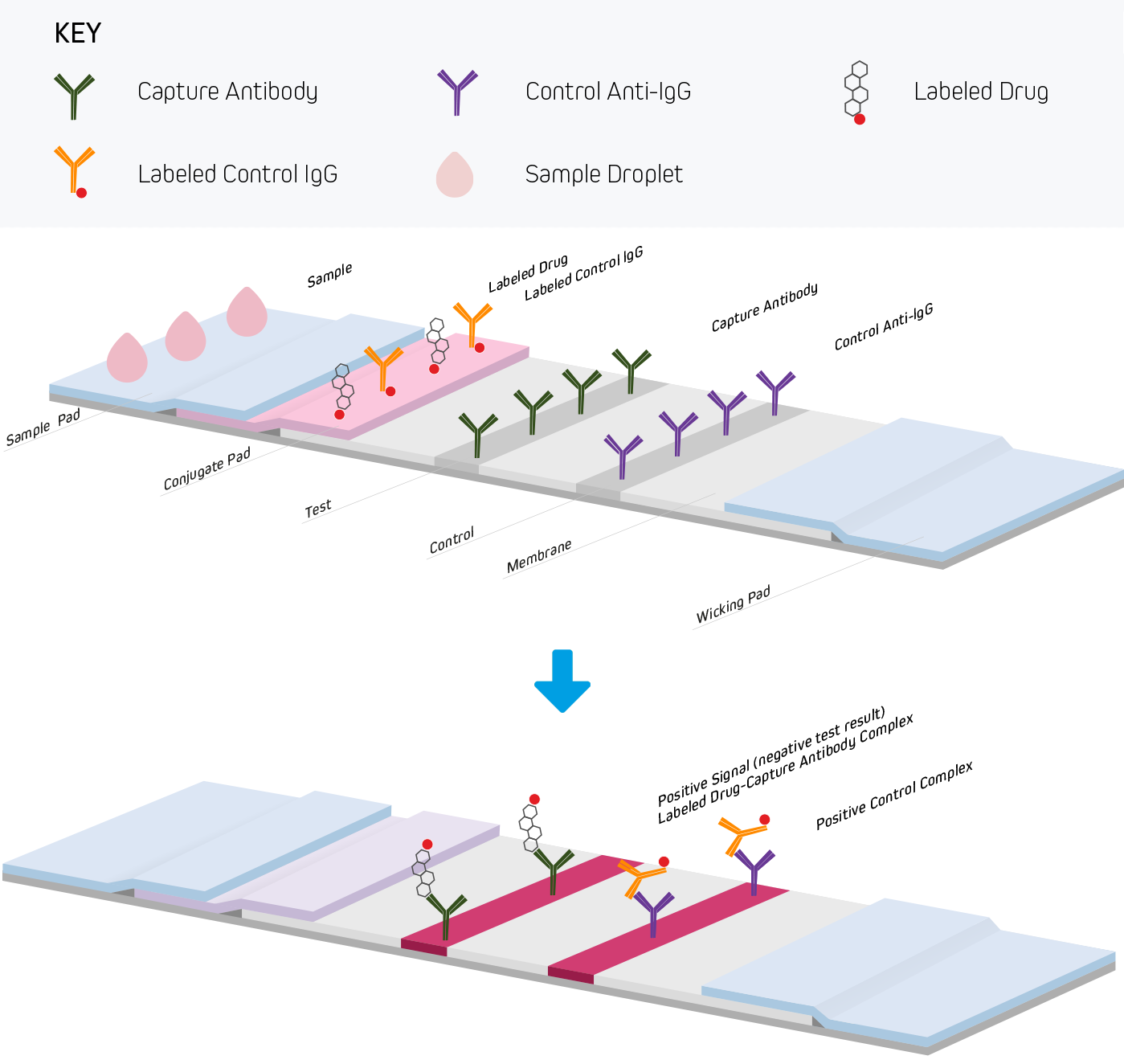
Lateral Flow Immunoassays Jackson Immunoresearch
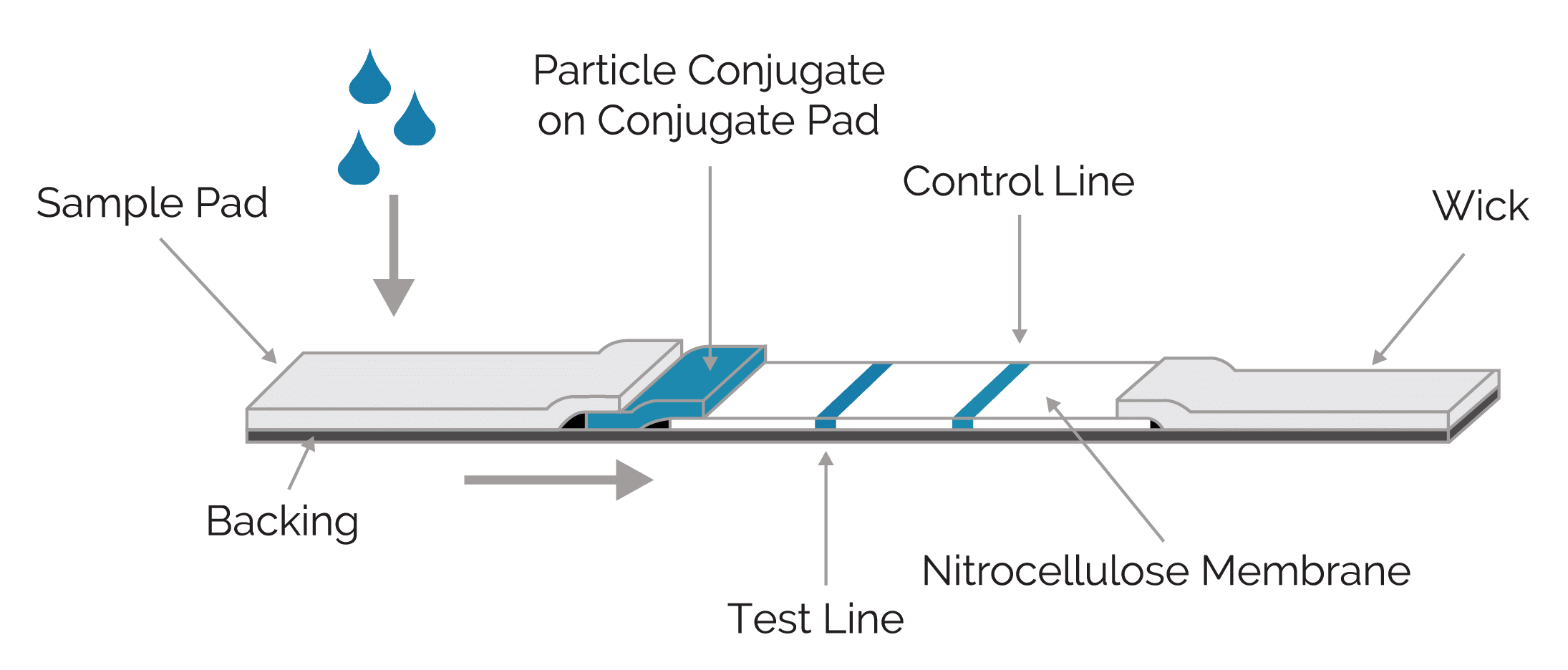
Lateral Flow Assays How Does Lateral Flow Work Dcn Dx

Public Health Agency What S The Difference Between A Pcr And Lateral Flow Test Which Test Do I Need And When Find Out More About Covid 19 Tests At Www Pha Site Cvtesting Facebook
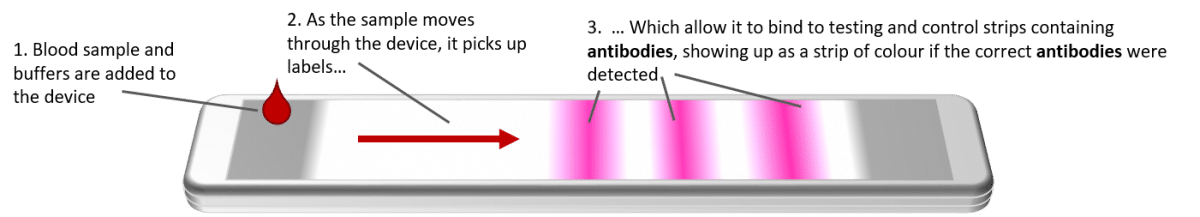
What Tests Could Potentially Be Used For The Screening Diagnosis And Monitoring Of Covid 19 And What Are Their Advantages And Disadvantages The Centre For Evidence Based Medicine
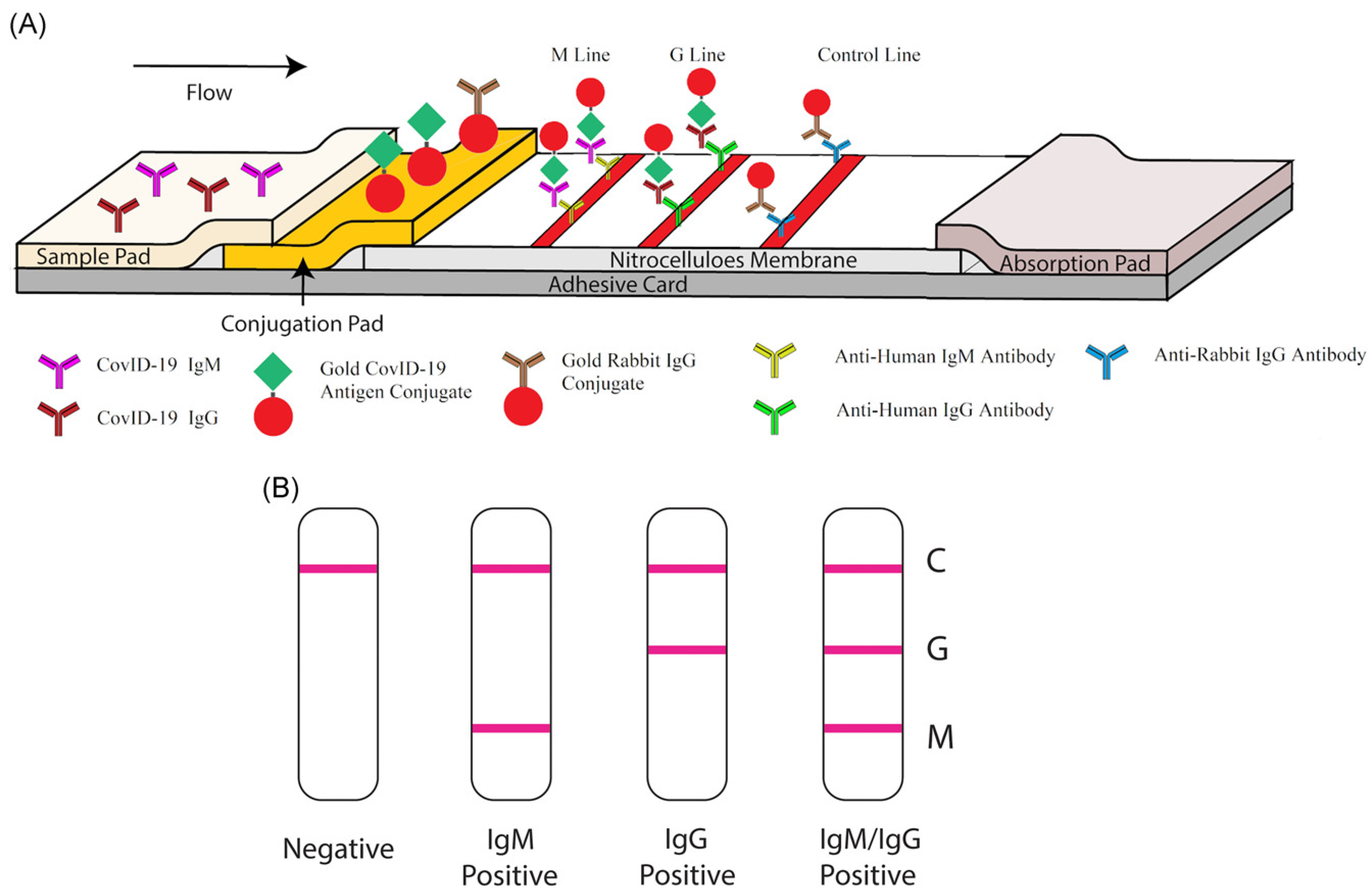
Biosensors Free Full Text Recent Advances In Novel Lateral Flow Technologies For Detection Of Covid 19 Html